Cutaneous squamous cell carcinoma of the head and neck (cSCCHN) is the most frequent cutaneous malignancy leading to perineural spread (PNS). This clinicoradiologic finding is associated with malignant infiltration of the perineural space of large caliber, named nerves with retrograde spread away from the primary site. This results in neural dysfunction and central failure at the brainstem if left untreated. This entity is defined by both abnormal neural findings on imaging as well as clinical neural dysfunction. In the head and neck, the trigeminal and facial nerve systems are the most commonly involved, with the latter being involved in 25%–35% of PNS cases. Often, there is concurrent involvement of both systems leading to facial paresis/paralysis as well as sensory disturbances1.
PNS should be distinguished from incidental perineural invasion (PNI) noted on routine histologic examination. PNI simply denotes tumor cell invasion into nearby small non-named nerves with no perineural spread or clinical dysfunction. Figures 1 and 2 demonstrate typical histologic pattens of PNS. Patients with cSCC-related PNS compared to cSCC-related PNI have higher overall risks of local recurrence and death and worse 5-year RFS and DSS2.
A high index of clinical suspicion is critical for PNS detection. Most cases present with incomplete, progressive facial paralysis worsening over weeks to months. This presentation distinguishes PNS-related facial palsy from benign paralysis etiologies as Bell’s palsy or Ramsay-Hunt Syndrome, which progressed to complete paralysis within 72 hours. Adding to diagnostic dilemma, many patients may not report a history of cSCCHN excision or may have a very remote history of excision. In the largest published series of cSCCHN PNS-related facial palsy, Schachtel et al. reported 89.0% of cases had recurrent disease at time of PNS diagnosis with the primary being treated on average almost 2 years prior. The mean duration from symptom onset to PNS diagnosis was 8.9 months (median, 6 months; range, 0.5–48 months) . In this same series, most presented with concurrent trigeminal involvement (67.1%) vs. isolated facial palsy1.
Magnetic resonance imaging (MRI) is the study of choice for PNS detection and defining the extent of retrograde spread. MRI has a reported sensitivity of 95% and specificity of 84% and 89% accuracy in identifying PNS zonal extent3. MRI findings may include: (1) Asymmetric neural thickening and/or enhancement (Fig. 3-4); (2) Obliteration of perineural fat pads (Fig. 5); and (3) Denervation changes (Fig. 6) 4. A zonal classification scheme for PNS was developed by Williams et al. (Fig. 7) and is utilized for treatment planning5. Regarding treatment strategies, primary surgery followed by post-operative radiotherapy are the mainstay modalities. Definitive surgery entails removing the involved nerve(s), its branches, and any associated tumor mass en bloc with clear margins. Particular attention is given to clear proximal neural margins to halt continued retrograde spread1.
In conclusion, a high index of clinical suspicion is required to recognize malignant facial palsy related to cSCCHN PNS. Updated preoperative MR imaging is critical to define the zonal extent of PNS, which will guide surgical planning. Further education of non-head and neck surgeons regarding facial palsy related to cSCCHN PNS is imperative to limit diagnostic and treatment delays and improve outcomes.
As a head and neck surgical oncologist and facial reanimation surgeon, I regularly receive referrals for “Bell’s palsy” which are quickly diagnosed as anything but based on the history alone. A history of an indolent, progressive facial paralysis with or without sensory disturbance occurring over the span of weeks to months should immediately raise a red flag for malignancy-related neuropathy. Most patients report a previous facial cSCC resection or cryotherapy but some patients deny previous facial skin cancer history. We follow the diagnostic and treatment algorithm proposed by Schachtel et al.1. Contrasted MR imaging with dedicated facial and trigeminal protocols are performed to define the zonal extent of PNS. Definitive surgery is offered for patients with radiologic zone 1 and 2 disease followed by postoperative radiotherapy.
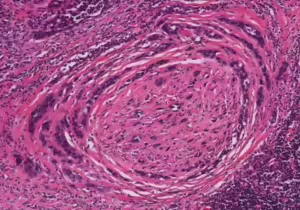
Figure 1. Perineural spread by carcinoma
H&E stain displaying characteristic onion skin pattern.
Photo c/o Kelly R. Magliocca, DDS, MPH
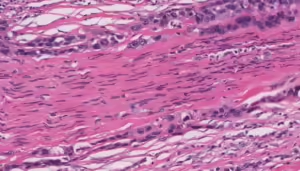
Figure 2. Perineural spread by carcinoma
H&E stain demonstrating longitudinal section with perineural spread
Photo c/o Kelly R. Magliocca, DDS, MPH
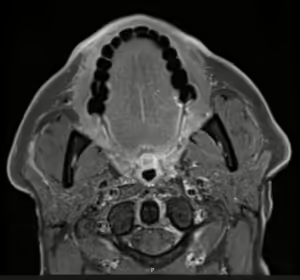
Figure 3. Asymmetric neural thickening and/or enhancement
Post-contrast thin-section axial T1-weighted fat-saturated MR image demonstrating linear enhancement then extends posteriorly along the right muscles of facial expression overlying the right masseter and a linear fashion into the substance of the right parotid gland along the expected course of the right facial nerve.
Photo c/o H. Michael Baddour, MD
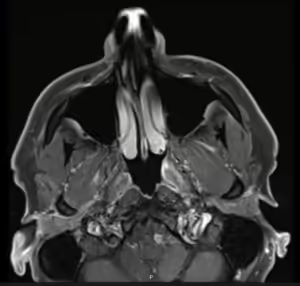
Figure 4. Asymmetric neural thickening and/or enhancement
Post-contrast thin-section axial T1-weighted fat-saturated MR image demonstrating asymmetric enhancement along the course of the right facial nerve at the stylomastoid foramen.
Photo c/o H. Michael Baddour, MD
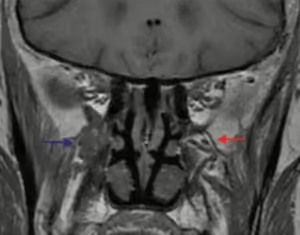
Figure 5. Obliteration of perineural fat pads
Coronal T1 MR image demonstrates the normal fat pad in the left pterygopalatine (PG) fossa (red arrow) as compared with tumor infiltration of the right PG fossa (blue arrow).
Image and caption adapted from: Gandhi M, Sommerville J. The Imaging of Large Nerve Perineural Spread. J Neurol Surg B Skull Base. 2016 Apr;77(2):113-23.
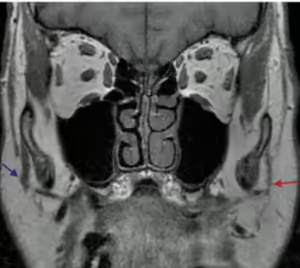
Figure 6. Denervation changes
Coronal T1 MR image demonstrates chronic denervation change in the distribution of the facial nerve with atrophy of the muscles of facial expression on the left (red arrow).
Image and caption adapted from: Gandhi M, Sommerville J. The Imaging of Large Nerve Perineural Spread. J Neurol Surg B Skull Base. 2016 Apr;77(2):113-23.
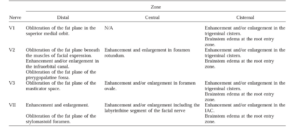
Figure 7. Imaging findings of perineural spread involving V1-V3 and VII
Adapted from: Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001 Mar 15;49(4):1061-9.
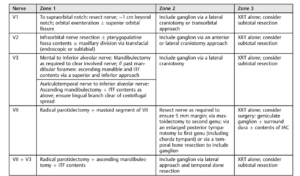
Figure 8. Surgical management of perineural spread
Adapted from: Panizza B, Warren T. Perineural invasion of head and neck skin cancer: diagnostic and therapeutic implications. Curr Oncol Rep. 2013 Apr;15(2):128-33. doi: 10.1007/s11912-012-0288-y. PMID: 23269602.
References:
- Schachtel MJC, Gandhi M, Bowman JJ, Porceddu SV, Panizza BJ. Facial nerve perineural spread from cutaneous squamous cell carcinoma of the head and neck: A single institution analysis of epidemiology, treatment, survival outcomes, and prognostic factors. Head Neck. 2022 May;44(5):1223-1236.
- Karia PS, Morgan FC, Ruiz ES, Schmults CD. Clinical and Incidental Perineural Invasion of Cutaneous Squamous Cell Carcinoma: A Systematic Review and Pooled Analysis of Outcomes Data. JAMA Dermatol. 2017 Aug 1;153(8):781-788
- Baulch J, Gandhi M, Sommerville J, Panizza B. 3T MRI evaluation of large nerve perineural spread of head and neck cancers. J Med Imaging Radiat Oncol. 2015Oct;59(5):578-85
- Gandhi M, Sommerville J. The Imaging of Large Nerve Perineural Spread. J Neurol Surg B Skull Base. 2016 Apr;77(2):113-23.
- Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001 Mar 15;49(4):1061-9.
- Panizza B, Warren T. Perineural invasion of head and neck skin cancer: diagnostic and therapeutic implications. Curr Oncol Rep. 2013 Apr;15(2):128-33. doi: 10.1007/s11912-012-0288-y. PMID: 23269602.